Which Describes Bonding Electrons in a Polar Covalent Bond
The two atoms in the bond have an electronegativity difference of greater than 18. ACTIVE LEARNING ACTIVITY Bond Covalent Bond Directions for the Student.
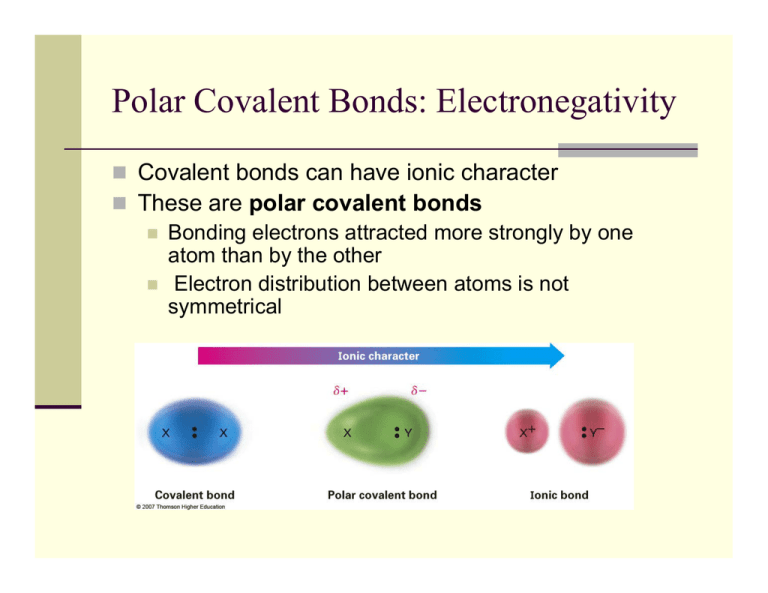
Polar Covalent Bonds Electronegativity
The bond dipole is zero.

. Which statements correctly describe a polar covalent bond. Nonpolar covalent bond - If there is no diff erence in electronegativity between the two atoms the bond is considered nonpolar covalent. Electrons are shared but not equally by the two atoms.
What are the properties of covalent bonds. When two atoms share one pair of electrons the bond is called a _______ bond. I and III only b.
A polar bond is a covalent bond in which electrons are shared equally. Covalent molecules with this kind of uneven charge distribution are polar. In a polar covalent bond the atom with the lower electronegativity is marked δ- because it has less electron density.
Which of the following best describes a polar covalent bond. Polar covalent bonds form when two atoms share electrons equally with electrons spend the same amount of time around each atom and no partial charges are formed. When the difference in electronegativity values is bigger than 05 the bond formed.
Which statements correctly describe a polar covalent bond. Polar covalent bonding is a type of chemicalbond where a pair of electrons is unequally shared between two atoms. What is polar covalent bonding.
Covalent bonds can form between two nonmetal atoms. Describe how covalent bonds are formed. The two atoms in the bond have an electronegativity difference of between 04 and 1 A metal bonding.
A chemical bond that involves sharing a pair of electrons between atoms in a molecule. One atom has a small negative charge and the other atom has a small positive charge. A ______ is a neutral group of atoms that are joined.
Which of the following describe a polar covalent bond. Molecules with polar covalent bonds have a positive and negative view. In a polar covalent bond the electron density is highest near the more electronegative atom.
A covalent bond in which the electrons are shared equally by the two atoms Polar Covalent Bond a covalent bond between atoms in which the electrons are shared unequally. A fluorine atom has seven valence electrons. Use your notes and follow along in the text as you find necessary.
Polar covalent bond - Th e electrons in a polar bond tend to spend more time around the more electronegative atom. For this type of bond the bonding electrons will be shared equally among those respective atoms which means that the electron density will not be greater for one or the other. Electrons that are not shared equally electrons that are shared equally el.
One atom has a small negative charge and the other atom has a small positive charge. Electrons in the bond are shared equally by the atoms. A _______ bond is a chemical bond in which two atoms share a pair of valence electrons.
The bonding of hydrogen and chlorine atoms leans more towards Cl atoms because Cl is. The electrons spend a longer time around some atoms than the. The bond dipole is zero.
Electrons are shared but the shared electrons came entirely from one of the bonded atoms c. In a polar covalent bond the electrons are not equally shared because one atom spends more time with the. Polar covalent bond is a type of chemical bond where one pair of electrons is shared unevenly between two atoms.
You could say that the two bonding electrons will spend an equal amount of time on each of the two atoms. Which statement below best describes a polar covalent bond. I and II only d.
Electrons are transferred completely from one atom to another b. Covalent bonds space a course of chemical bonds wherein valence electrons are shared between two atoms typically two nonmetals. Which is more polar a polar covalent bond or an ionic bond - soft - poor conductors of heat and electronegativity - low melting and boiling point.
Forming Covalent Bonds. Correct answer - Which describes bonding electrons in a polar covalent bond. In this type electrons are shared equally by each molecule.
An _____ is the smallest particle of a covalent compound that still has the properties of the compound. Which statement describes a multiple covalent bond. In a polar covalent bond what do each side get.
Electrons are shared but not equally by the two atoms. Which statement best describes a covalent bond. This lesson is designed for you to complete on your own or in your study group.
The formation of a covalent bond permits the nonmetals to obey the octet rule and thus become much more stable. A polar covalent bond is when one atom holds one or more of the sharing electrons closer to itself ie. Electrons are shared but are pulled more strongly towardby one of the atoms d.
List all that apply. One or more electrons are transferred from one atom to a second atom. One or more electrons are transferred from one atom to a second atom.
Differentiate between a polar covalent bond and a non-polar covalent bond. A non-metal bonding to a non-metal. Polar Covalent Bond Definition Polar Covalent Bond Definition A polar bond may be a chemical bond among two atoms where the electrons build the bond are unfairly shared.
For example Hydrogen chloride HCl molecules. Polar covalent bonds form when two atoms share electrons unequally with one electron spending more time around one atom than.

Polar Covalent Bond Definition And Examples
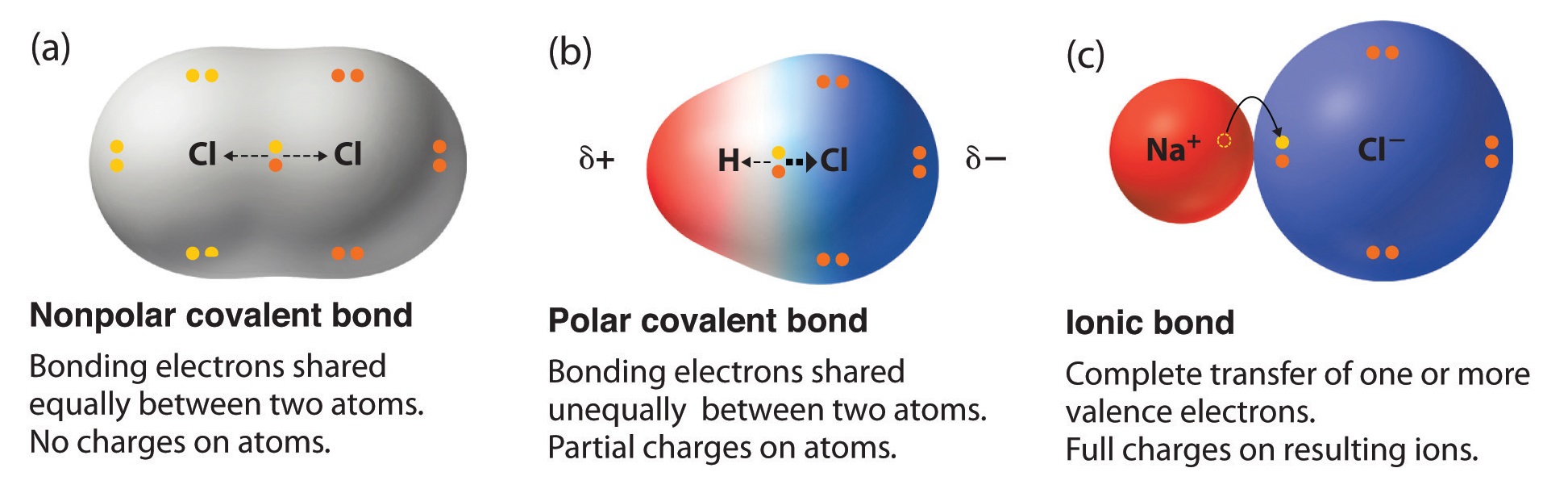
9 3 Molecular Shape And Molecular Polarity Chemistry Libretexts

No comments for "Which Describes Bonding Electrons in a Polar Covalent Bond"
Post a Comment